Whay is Alcoholic?
What is Alcohol?
Historically, alcohol has been used in association with social activities, including both religious and non-religious rituals, as a dietary component, and as a medicinal agent. Alcohol consumption by various cultures predates written history. Although it was once used for therapeutic purposes, it is no longer recommended as a therapeutic because of its ability to produce intoxication. The ability of ingested alcohol to get from the gut into the bloodstream and up to the brain where it produces the intoxicating effects is due to its chemical structure and solubility in water.
Historically, alcohol has been used in association with social activities, including both religious and non-religious rituals, as a dietary component, and as a medicinal agent. Alcohol consumption by various cultures predates written history. Although it was once used for therapeutic purposes, it is no longer recommended as a therapeutic because of its ability to produce intoxication. The ability of ingested alcohol to get from the gut into the bloodstream and up to the brain where it produces the intoxicating effects is due to its chemical structure and solubility in water.
Chemical structure of alcohol
Alcohols are organic molecules assembled from carbon (C), oxygen (O), and hydrogen (H) atoms. When 2 carbons are present, the alcohol is called ethanol (also known as ethyl alcohol). Ethanol is the form of alcohol contained in beverages including beer, wine, and liquor.
Learn more about the formation of alcohol in beverages.
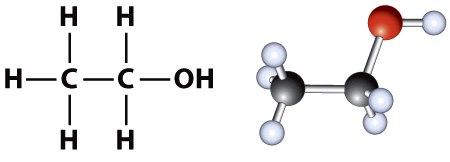
The chemical composition of ethanol can be represented either as a 1) molecular formula or as a 2) structural formula. The molecular formula of ethanol is C2H6O, indicating that ethanol contains two carbons and an oxygen. However, the structural formula of ethanol, C2H5OH, provides a little more detail, and indicates that there is an hydroxyl group (-OH) at the end of the 2-carbon chain (Figure 1.1). The -OH group is characteristic of all alcohols.

Figure 1.1 Two common ways to represent the structure of ethanol are shown. On the left is the atomic stick representation of the structural formula and on the right is the ball and stick model.
Alcohols are organic molecules assembled from carbon (C), oxygen (O), and hydrogen (H) atoms. When 2 carbons are present, the alcohol is called ethanol (also known as ethyl alcohol). Ethanol is the form of alcohol contained in beverages including beer, wine, and liquor.
Learn more about the formation of alcohol in beverages.
The chemical composition of ethanol can be represented either as a 1) molecular formula or as a 2) structural formula. The molecular formula of ethanol is C2H6O, indicating that ethanol contains two carbons and an oxygen. However, the structural formula of ethanol, C2H5OH, provides a little more detail, and indicates that there is an hydroxyl group (-OH) at the end of the 2-carbon chain (Figure 1.1). The -OH group is characteristic of all alcohols.
Figure 1.1 Two common ways to represent the structure of ethanol are shown. On the left is the atomic stick representation of the structural formula and on the right is the ball and stick model.
Ethanol is soluble in water
Ethanol is an interesting molecule. It is polar or hydrophilic (water-loving) due to the presence of the terminal hydroxyl group, so it dissolves in water. Yet because of the 2 carbon chain, it has a bit of non-polar character. There is no separation of electrical charges between the carbon atoms, thereby minimizing intermolecular interactions in aqueous solutions. Generally, carbon chains (saturated with hydrogens) give a molecule hydrophobic (water-fearing) character, making it less soluble in water. However, in the case of ethanol, the carbon chain is short enough so that the more polar -OH group dominates, giving the ethanol its polar character. In alcohols with relatively long carbon chains (4 or more), the polar effects of the -OH group are not sufficient to overcome the hydrophobic nature of the carbon chain, resulting in alcohols that are progressively less water-soluble.
The solubility characteristics of ethanol become important in terms of its ability to move across biological membranes and around the body. Because it is a small molecule (molecular weight = 46 g/mole), it fits through pores (holes) in the biological membrane. In fact, it distributes in any area within the body in which water is found. However, the 2-carbon chain in ethanol makes it slightly lipophilic (lipid-loving) so it can also penetrate the lipid bilayers of biological membranes.
 Figure 1.2 The solubility of an alcohol depends on the presence of a terminal hydroxyl (OH) group, and the length of its carbon chain.
Figure 1.2 The solubility of an alcohol depends on the presence of a terminal hydroxyl (OH) group, and the length of its carbon chain.
Ethanol is an interesting molecule. It is polar or hydrophilic (water-loving) due to the presence of the terminal hydroxyl group, so it dissolves in water. Yet because of the 2 carbon chain, it has a bit of non-polar character. There is no separation of electrical charges between the carbon atoms, thereby minimizing intermolecular interactions in aqueous solutions. Generally, carbon chains (saturated with hydrogens) give a molecule hydrophobic (water-fearing) character, making it less soluble in water. However, in the case of ethanol, the carbon chain is short enough so that the more polar -OH group dominates, giving the ethanol its polar character. In alcohols with relatively long carbon chains (4 or more), the polar effects of the -OH group are not sufficient to overcome the hydrophobic nature of the carbon chain, resulting in alcohols that are progressively less water-soluble.
The solubility characteristics of ethanol become important in terms of its ability to move across biological membranes and around the body. Because it is a small molecule (molecular weight = 46 g/mole), it fits through pores (holes) in the biological membrane. In fact, it distributes in any area within the body in which water is found. However, the 2-carbon chain in ethanol makes it slightly lipophilic (lipid-loving) so it can also penetrate the lipid bilayers of biological membranes.Figure 1.2 The solubility of an alcohol depends on the presence of a terminal hydroxyl (OH) group, and the length of its carbon chain.
Alcoholic drink
An alcoholic drink (or alcoholic beverage) is a drink that contains ethanol, a type of alcohol produced by fermentation of grains, fruits, or other sources of sugar. The consumption of alcohol plays an important social role in many cultures. Most countries have laws regulating the production, sale, and consumption of alcoholic beverages.[2] Some countries ban such activities entirely, but alcoholic drinks are legal in most parts of the world. The global alcoholic drink industry exceeded $1 trillion in 2018.[1]


Alcohol is a depressant, which in low doses causes euphoria, reduces anxiety, and increases sociability. In higher doses, it causes drunkenness, stupor, unconsciousness, or death. Long-term use can lead to alcohol abuse, cancer, physical dependence, and alcoholism.
Alcohol is one of the most widely used recreational drugs in the world, with about 33% of people being current drinkers.[3] As of 2016, women on average drink 0.7 drinks and males 1.7 drinks a day.[3] In 2015, among Americans, 86% of adults had consumed alcohol at some point, with 70% drinking it in the last year and 56% in the last month.[4] Alcoholic drinks are typically divided into three classes—beers, wines, and spirits—and typically their alcohol content is between 3% and 50%.
Discovery of late Stone Age jugs suggest that intentionally fermented drinks existed at least as early as the Neolithic period (c. 10,000 BC).[5] Several animals (but not all) are affected by alcohol similarly to humans and, once they consume it, will consume it again if given the opportunity, though humans are the only species known to produce alcoholic drinks intentionally.[6]
Fermented drinks
Beer
Beer is a beverage fermented from grain mash. It is typically made from barley or a blend of several grains and flavored with hops. Most beer is naturally carbonated as part of the fermentation process. If the fermented mash is distilled, then the drink becomes a spirit. In the Andean region, the most common beer is chicha, made from grain or fruits.[7] Beer is the most consumed alcoholic beverage in the world.[8]
Wine
Wine is a fermented beverage produced from grapes and sometimes other fruits. Wine involves a longer fermentation process than beer and a long aging process (months or years), resulting in an alcohol content of 9%–16% ABV.
Cider
Cider or cyder (/ˈsaɪdər/ SY-dər) is a fermented alcoholic drink made from any fruit juice; apple juice (traditional and most common), peaches, pears ("Perry" cider) or other fruit. Cider alcohol content varies from 1.2% ABV to 8.5% or more in traditional English ciders. In some regions, cider may be called "apple wine".[9]
Fermented tea
Fermented tea (also known as post-fermented tea or dark tea) is a class of tea that has undergone microbial fermentation, from several months to many years. The tea leaves and the liquor made from them become darker with oxidation. Thus, the various kinds of fermented teas produced across China are also referred to as dark tea, not be confused with black tea. The most famous fermented tea is kombucha which is often homebrewed, pu-erh, produced in Yunnan Province,[10][11] and the Anhua dark tea produced in Anhua County of Hunan Province. The majority of kombucha on the market are under 0.5% ABV.
Mead
Mead (/miːd/) is an alcoholic drink made by fermenting honey with water, sometimes with various fruits, spices, grains, or hops. The alcoholic content of mead may range from about 8% ABV to more than 20%. The defining characteristic of mead is that the majority of the drink's fermentable sugar is derived from honey.
Pulque
Pulque is the Mesoamerican fermented drink made from the "honey water" of maguey, Agave americana. The drink distilled from pulque is tequila or mescal Mezcal.[12]
Rice wine
Sake, huangjiu, mijiu, and cheongju are popular examples of East Asian rice wine.
Others
"Fruit wines" are made from fruits other than grapes, such as plums, cherries, or apples.
Sparkling wine like French Champagne, Catalan Cava or Italian Prosecco can be made by means of a secondary fermentation.
Distilled drinks
A distilled drink or liquor is an alcoholic drink produced by distilling (i.e., concentrating by distillation) ethanol produced by means of fermenting grain, fruit, or vegetables.[13] Unsweetened, distilled, alcoholic drinks that have an alcohol content of at least 20% ABV are called spirits.[14] For the most common distilled drinks, such as whiskey and vodka, the alcohol content is around 40%. The term hard liquor is used in North America to distinguish distilled drinks from undistilled ones (implicitly weaker). Vodka, gin, baijiu, shōchū, soju, tequila, whiskey, brandy and rum are examples of distilled drinks. Distilling concentrates the alcohol and eliminates some of the congeners. Freeze distillation concentrates ethanol along with methanol and fusel alcohols (fermentation by-products partially removed by distillation) in applejack.
Fortified wine is wine, such as port or sherry, to which a distilled beverage (usually brandy) has been added.[15] Fortified wine is distinguished from spirits made from wine in that spirits are produced by means of distillation, while fortified wine is simply wine that has had a spirit added to it. Many different styles of fortified wine have been developed, including port, sherry, madeira, marsala, commandaria, and the aromatized wine vermouth.[16]
Rectified spirit
Rectified spirit, also called "neutral grain spirit", is alcohol which has been purified by means of "rectification" (i.e. repeated distillation). The term neutral refers to the spirit's lack of the flavor that would have been present if the mash ingredients had been distilled to a lower level of alcoholic purity. Rectified spirit also lacks any flavoring added to it after distillation (as is done, for example, with gin). Other kinds of spirits, such as whiskey, are distilled to a lower alcohol percentage to preserve the flavor of the mash.
Rectified spirit is a clear, colorless, flammable liquid that may contain as much as 95% ABV. It is often used for medicinal purposes. It may be a grain spirit or it may be made from other plants. It is used in mixed drinks, liqueurs, and tinctures, and also as a household solvent.
Congeners
In the alcoholic drinks industry, congeners are substances produced during fermentation. These substances include small amounts of chemicals such as occasionally desired other alcohols, like propanol and 3-methyl-1-butanol, but also compounds that are never desired such as acetone, acetaldehyde and glycols. Congeners are responsible for most of the taste and aroma of distilled alcoholic drinks, and contribute to the taste of non-distilled drinks.[17] It has been suggested that these substances contribute to the symptoms of a hangover.[18] Tannins are congeners found in wine in the presence of phenolic compounds. Wine tannins add bitterness, have a drying sensation, taste herbaceous and are often described as astringent. Wine tannins adds balance, complexity, structure and makes a wine last longer, so they play an important role in the aging of wine.[19]
Food energy
Alcoholic drinks are a source of food energy. The USDA uses a figure of 6.93 kilocalories (29.0 kJ) per gram of alcohol (5.47 kcal or 22.9 kJ per ml) for calculating food energy.[20] In addition to alcohol, many alcoholic drinks contain carbohydrates. For example, in 12 US fl oz (355 ml) of 5% ABV beer, along with approximately 18 ml of alcohol (96 kilocalories or 400 kilojoules), there are usually 10–15 g of carbohydrates (about 40–60 kcal or 170–250 kJ).[citation needed] Excessive daily calorie intake may contribute to an increase in body weight and "beer belly". In addition to the direct effect of its caloric content, alcohol is also known to potentiate the insulin response of the human body to glucose, which, in essence, "instructs" the body to convert consumed carbohydrates into fat and to suppress carbohydrate and fat oxidation.[21][22] Ethanol is directly processed in the liver to acetyl CoA, the same intermediate product as in glucose metabolism. Because ethanol is mostly metabolized and consumed by the liver, chronic excessive use can lead to fatty liver. This leads to a chronic inflammation of the liver and eventually alcoholic liver disease.
Amount of use

The average number of people who drink as of 2016 was 39% for males and 25% for females (2.4 billion people in total).[3] Females on average drink 0.7 drinks per day while males drink 1.7 drinks per day.[3] The rates of drinking varies significantly in different areas of the world.[3]
Reasons for use
Apéritifs and digestifs
An apéritif is any alcoholic beverage usually served before a meal to stimulate the appetite,[24] while a digestif is any alcoholic beverage served after a meal for the stated purpose of improving digestion. Fortified wine, liqueurs, and dry champagne are common apéritifs. Because apéritifs are served before dining, they are usually dry rather than sweet. One example is Cinzano, a brand of vermouth. Digestifs include brandy, fortified wines and herb-infused spirits (Drambuie).
Flavoring

Pure ethanol tastes bitter to humans; some people also describe it as sweet.[25] However, ethanol is also a moderately good solvent for many fatty substances and essential oils. This facilitates the use of flavoring and coloring compounds in alcoholic drinks as a taste mask, especially in distilled drinks. Some flavors may be naturally present in the beverage's raw material. Beer and wine may also be flavored before fermentation, and spirits may be flavored before, during, or after distillation. Sometimes flavor is obtained by allowing the beverage to stand for months or years in oak barrels, usually made of American or French oak. A few brands of spirits may also have fruit or herbs inserted into the bottle at the time of bottling.
Wine is important in cuisine not just for its value as an accompanying beverage, but as a flavor agent, primarily in stocks and braising, since its acidity lends balance to rich savory or sweet dishes.[26] Wine sauce is an example of a culinary sauce that uses wine as a primary ingredient.[27] Natural wines may exhibit a broad range of alcohol content, from below 9% to above 16% ABV, with most wines being in the 12.5–14.5% range.[28] Fortified wines (usually with brandy) may contain 20% alcohol or more.
Alcohol measurement
Alcohol concentration
The concentration of alcohol in a beverage is usually stated as the percentage of alcohol by volume (ABV, the number of milliliters (ml) of pure ethanol in 100 ml of beverage) or as proof. In the United States, proof is twice the percentage of alcohol by volume at 60 degrees Fahrenheit (e.g. 80 proof = 40% ABV). Degrees proof were formerly used in the United Kingdom, where 100 degrees proof was equivalent to 57.1% ABV. Historically, this was the most dilute spirit that would sustain the combustion of gunpowder.
Ordinary distillation cannot produce alcohol of more than 95.6% by weight, which is about 97.2% ABV (194.4 proof) because at that point alcohol is an azeotrope with water. A spirit which contains a very high level of alcohol and does not contain any added flavoring is commonly called a neutral spirit. Generally, any distilled alcoholic beverage of 170 US proof or higher is considered to be a neutral spirit.[30]
Most yeasts cannot reproduce when the concentration of alcohol is higher than about 18%, so that is the practical limit for the strength of fermented drinks such as wine, beer, and sake. However, some strains of yeast have been developed that can reproduce in solutions of up to 25% ABV.[31]
Serving measures
Shot sizes
Shot sizes vary significantly from country to country. In the United Kingdom, serving size in licensed premises is regulated under the Weights and Measures Act (1985). A single serving size of spirits (gin, whisky, rum, and vodka) are sold in 25 ml or 35 ml quantities or multiples thereof.[32] Beer is typically served in pints (568 ml), but is also served in half-pints or third-pints. In Israel, a single serving size of spirits is about twice as much, 50 or 60 mL.
The shape of a glass can have a significant effect on how much one pours. A Cornell University study of students and bartenders' pouring showed both groups pour more into short, wide glasses than into tall, slender glasses.[33] Aiming to pour one shot of alcohol (1.5 ounces or 44.3 ml), students on average poured 45.5 ml & 59.6 ml (30% more) respectively into the tall and short glasses. The bartenders scored similarly, on average pouring 20.5% more into the short glasses. More experienced bartenders were more accurate, pouring 10.3% less alcohol than less experienced bartenders. Practice reduced the tendency of both groups to over pour for tall, slender glasses but not for short, wide glasses. These misperceptions are attributed to two perceptual biases: (1) Estimating that tall, slender glasses have more volume than shorter, wider glasses; and (2) Over focusing on the height of the liquid and disregarding the width.
Standard drinks

A standard drink is a notional drink that contains a specified amount of pure alcohol. The standard drink is used in many countries to quantify alcohol intake. It is usually expressed as a measure of beer, wine, or spirits. One standard drink always contains the same amount of alcohol regardless of serving size or the type of alcoholic beverage. The standard drink varies significantly from country to country. For example, it is 7.62 ml (6 grams) of alcohol in Austria, but in Japan it is 25 ml (19.75 grams).
- In the United Kingdom, there is a system of units of alcohol which serves as a guideline for alcohol consumption. A single unit of alcohol is defined as 10 ml. The number of units present in a typical drink is sometimes printed on bottles. The system is intended as an aid to people who are regulating the amount of alcohol they drink; it is not used to determine serving sizes.
- In the United States, the standard drink contains 0.6 US fluid ounces (18 ml) of alcohol. This is approximately the amount of alcohol in a 12-US-fluid-ounce (350 ml) glass of beer, a 5-US-fluid-ounce (150 ml) glass of wine, or a 1.5-US-fluid-ounce (44 ml) glass of a 40% ABV (80 US proof) spirit.
Laws
Alcohol laws regulate the manufacture, packaging, labelling, distribution, sale, consumption, blood alcohol content of motor vehicle drivers, open containers, and transportation of alcoholic drinks. Such laws generally seek to reduce the adverse health and social impacts of alcohol consumption. In particular, alcohol laws set the legal drinking age, which usually varies between 16 and 25 years, sometimes depending upon the type of drink (e.g., beer vs. hard liquor). Some countries do not have a legal drinking or purchasing age, but most countries set the minimum age at 18 years.[2] Some countries, such as the U.S., have the drinking age higher than the legal age of majority (18), at age 21 in all 50 states. Such laws may take the form of permitting distribution only to licensed stores, monopoly stores, or pubs and they are often combined with taxation, which serves to reduce the demand for alcohol (by raising its price) and it is a form of revenue for governments. These laws also often limit the hours or days (e.g., "blue laws") on which alcohol may be sold or served, as can also be seen in the "last call" ritual in US and Canadian bars, where bartenders and servers ask patrons to place their last orders for alcohol, due to serving hour cutoff laws. In some countries, alcohol cannot be sold to a person who is already intoxicated. Alcohol laws in many countries prohibit drunk driving.
In some jurisdictions, alcoholic drinks are totally prohibited for reasons of religion (e.g., Islamic countries with sharia law) or for reasons of local option, public health, and morals (e.g., Prohibition in the United States from 1920 to 1933). In jurisdictions which enforce sharia law, the consumption of alcoholic drinks is an illegal offense,[34] although such laws may exempt non-Muslims.[35]
History

- 10,000–5000 BC: Discovery of late Stone Age jugs suggests that intentionally fermented drinks existed at least as early as the Neolithic period.[36]
- 7000–5600 BC: Examination and analysis of ancient pottery jars from the neolithic village of Jiahu in the Henan province of northern China revealed residue left behind by the alcoholic drinks they had once contained. According to a study published in the Proceedings of the National Academy of Sciences, chemical analysis of the residue confirmed that a fermented drink made of grape and hawthorn fruit wine, honey mead and rice beer was being produced in 7000–5600 BC (McGovern et al., 2005; McGovern 2009).[37][38] The results of this analysis were published in December 2004.[39]
- 9th century AD: The medieval Arabs used the distillation process extensively, and applied it to the distillation of alcohol. The Arab chemist Al-Kindi unambiguously described the distillation of wine in the 9th century.[40][41][42]
- 12th century: The process of distillation spread from the Middle East to Italy,[40][43] where distilled alcoholic drinks were recorded in the mid-12th century.[44] In China, archaeological evidence indicates that the true distillation of alcohol began during the 12th century Jin or Southern Song dynasties.[45] A still has been found at an archaeological site in Qinglong, Hebei, dating to the 12th century.[45]
- 14th century: In India, the true distillation of alcohol was introduced from the Middle East, and was in wide use in the Delhi Sultanate by the 14th century.[43] By the early 14th century, distilled alcoholic drinks had spread throughout the European continent.[44]




Comments
Post a Comment